
With fertilization and implantation, the corpus luteum is maintained by stimulation of human chorionic gonadotropin (hCG) by the placenta.

At the luteal-follicular transition, FSH levels increase, and the next menstrual cycle begins 1. When inhibin and progesterone levels fall with regression of the corpus luteum, suppression of FSH is released. With no fertilization or implantation of the embryo, the corpus luteum degenerates, possibly in response to activin, homodimers (βA:βA or βB:βB) or heterodimers (βA:βB) that share the β subunits with inhibin A and inhibin B (α:βB). During the luteal phase, the granulosa cells within the corpus luteum also produce inhibin A, an α:βA heterodimeric member of the transforming growth factor β (TGFβ) superfamily, which acts as an endocrine hormone to suppresses pituitary FSH, inhibiting growth of other ovarian follicles. The remaining follicular cells in the ovary become luteinized as part of the corpus luteum which secretes progesterone. During ovulation, the oocyte is expulsed from the follicle with cumulus granulosa cells surrounding it. The very high estrogen levels feed back to the anterior pituitary to induce the LH surge, which ultimately leads to ovulation. This process selects for the dominant follicle 1. Follicles that are not at the appropriate stage and are not able to maintain a high estrogen microenvironment without stimulation from FSH degenerate and become atretic follicles. This high estrogen down regulates FSH from the anterior pituitary and begins the process of selecting for a single dominant follicle. This cross talk between the granulosa and thecal cells results in high estrogen levels within the follicle. At the pre-antral follicle stage, the follicle is a two cell (granulosa and thecal cells), two gonadotropin (FSH and LH) system. With an increase in estrogen, the antral follicle develops further. FSH induces granulosa cell proliferation, induction of aromatase, and increased FSH receptors on the granulosa cells, thus leading to a very high estrogen microenvironment. Androgens are converted to estrogen via a member of the cytochrome P450 superfamily, CYP19 (aromatase) in the granulosa cells. The theca, a layer of cells surrounding the follicle, is formed first at the two-layer pre-antral follicle stage, and with exposure to low levels of LH, produces androgens in humans. Without FSH, the follicles become atretic. In the presence of FSH, these follicles begin to grow even more and are competent to develop into an antral follicle. This cycle of folliculogenesis occurs for every single oocyte ovulated. Once the oocyte is ovulated, the remaining granulosa cells become the corpus luteum. this stage, cumulus and mural granulosa cells are present. The peri-ovulatory follicle stage is also known as the dominant follicle and is ready for ovulation. The early antral follicle stage is defined by the presence of antrum. At the secondary follicle stage, theca cells are present. The primordial follicles progress to primary and then secondary follicles. The process begins with the germ cells which are recruited to a pool of primordial follicles.

Folliculogenesis, ovulation, luteinization, and endometrium growth and decline during the menstrual cycle depend on the above-mentioned autocrine, paracrine, and endocrine factors produced from this axis 1.įolliculogenesis requires a coordinated progression of growth of ovarian follicles. Although estrogen and progesterone have some feedback at the level of the hypothalamus, the more dynamic feedback occurs at the level of the anterior pituitary. The ovarian steroid hormones in turn stimulate endometrial proliferation and affect many end organs. Similar to the pituitary, ovarian steroidogenesis is regulated by multiple factors. The gonadotropins stimulate the ovary to produce the steroid hormones, estrogen or progesterone as well as several key autocrine, paracrine, and endocrine peptides. The levels and timing of secretion of each gonadotropin is correlated by GnRH, feedback from sex steroid hormones, and other autocrine and paracrine factors such as inhibin and activin. The α subunit is common to all glycoprotein hormone family members. FSH and LH are heterodimeric members of the glycoprotein hormone family and have an α:FSHβ and α:LHβ non-covalent structure, respectively.
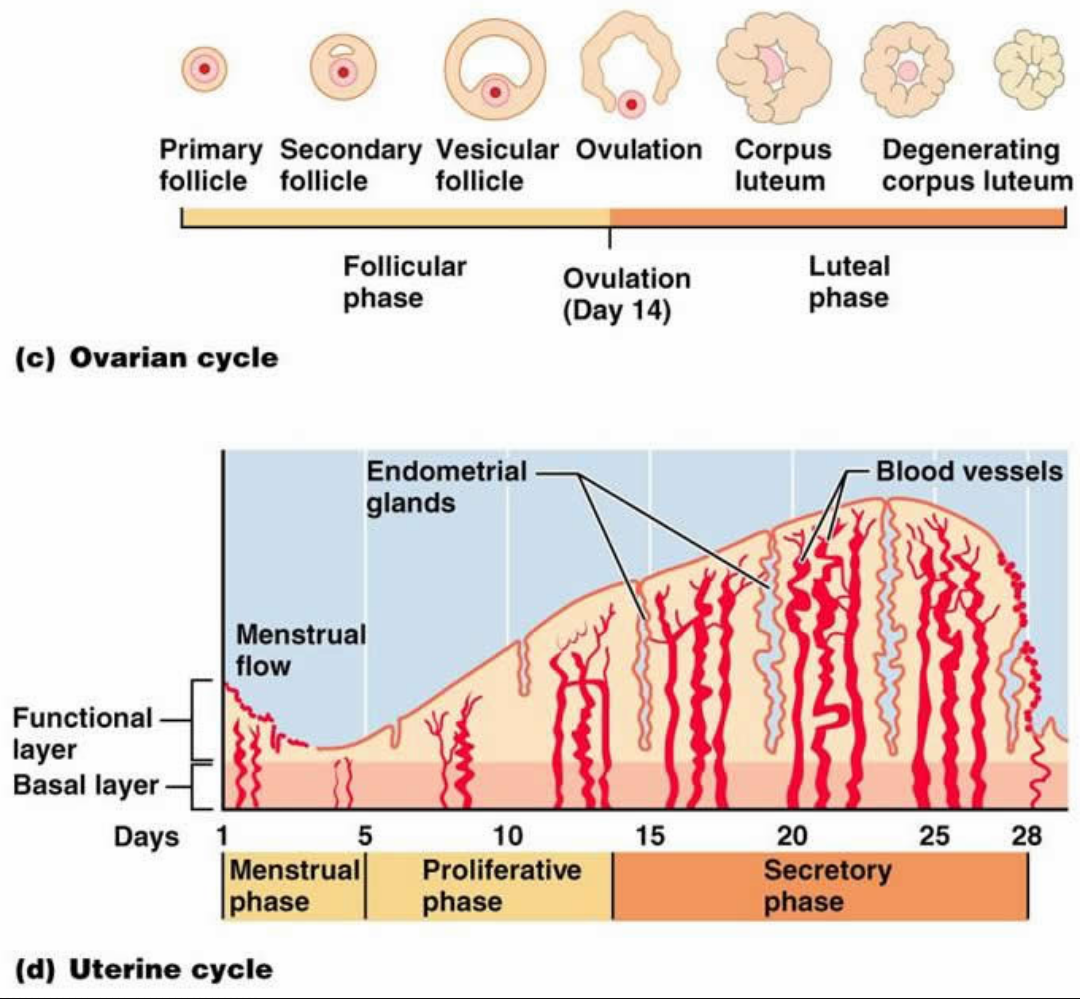
The hypothalamus secretes gonadotropin releasing hormone (GnRH) which stimulates the anterior pituitary to secrete both follicle stimulating hormone (FSH) and luteinizing hormone (LH). Overview of the Menstrual Cycle in Humansįigure 1 shows a general overview of the key regulatory factors in the menstrual cycle.


 0 kommentar(er)
0 kommentar(er)
